Thursday, August 29, 2019
Lupine Publishers: Soil Texture of Nesting Sites and Breeding Populat...
Lupine Publishers: Soil Texture of Nesting Sites and Breeding Populat...: Lupine Publishers- Environmental and Soil Science Abstract Investigating the breeding population and soil texture of breeding ...
Lupine Publishers: Change of Evaporations Leads to Climate Change
Lupine Publishers: Change of Evaporations Leads to Climate Change: Lupine Publishers- Environmental and Soil Science Short Communication The basis of all floods and droughts is water, its e...
Wednesday, August 28, 2019
Lupine publishers | Head Cancer and Metastasic Neck. What Have We Advanced in the Last Years?
Lupine Publishers | Open Access Journal of Oncology and Medicine
In 2012, 5.210 new cases of head and neck tumors were estimated in
the USA, with an increasing incidence due to tobacco and
alcohol habits in the population. A large percentage of the cases debut
as a locally advanced disease, so control of the disease is key
and we look for the best therapeutic strategy to achieve good survival
rates while maintaining quality of life. We present the case of
a 60-year-old patient in which our objectives to be presented are the
assessment of comorbidity, toxicity and survival.
A 60-year-old man without medical illnesses to be highlighted.
He came to the emergency room in July 2016 due to injury at the
cervical level of 1 month of evolution, with progressive growth and
breathness, with also difficulty for eating. Also asthenia, anorexia
and loss of weight not quantified in the last month.
Physical Examination: Weight 42 kg Head and Neck: mass of hard consistency of approximately 10 cm in diameter in the cervical left region that seems to deflect trachea. Pulmonary auscultation: generalized hypoventilation with some expiratory wheeze.
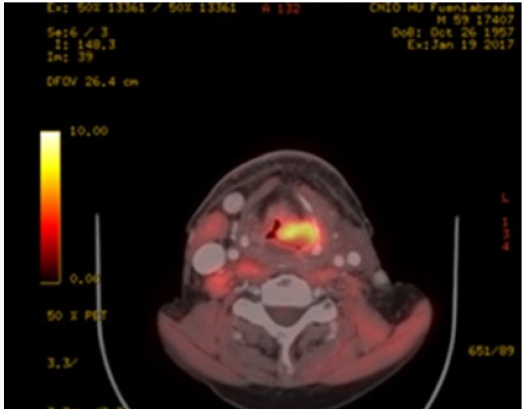
b. PET-CT (August 2016): Extensive tumor in pharynxlarynx- esophagus (> 6 cm) Lymph nodes in left IB-II spaces (> 6 cm). If confirmed, carcinoma would correspond to T3 N3 (stage IVB) (Figure 1).
c. Laryngeal Biopsy: Moderately differentiated and keratinizing squamous cell carcinoma.
So, confirmed Squamous cell carcinoma stage cT4N3 Mx.The case is presented in the Tumor Committee, deciding treatment with Chemoradiotherapy and 1 cycle of Docetaxel+ Cisplatin (60% dose reduction because of frailty/ malnutrition) previous to induction due to the large tumor volume and waiting for start radiotherapy. Tracheotomy is performed prior to starting because of the risk of airway obstruction and also gastrostomy is placed for nutritional support.
On 31th August 2016 the patient starts on RT concomitantly to Cisplain ( receiving 2 cycles on 12.09.2016 and 10.10.2016 and 70 Gy) During the treatment, he achieves a good general condition until January 2017, when he goes to Otolaryngology Clinics referring dysphagia again although he maintains weight in 51 kg. PET-CT is performed: disease progression with soft tissue increase in the pharyngoesophageal junction despite the good response of cervical adenopathies and the partial response of the primary tumor. New bilateral subpleural pulmonary nodules suggestive of metastasis. Figure 2 Due to progression, he restarts treatment with chemotherapy (palliative intention) with ERBITAX scheme (Paclitaxel 80 mg / m2 weekly (3 / 4s) + Cetuximab 400 mg / m2 followed by 250 mg / m2) with good tolerance,only highlighting secondary rash to cetuximab (predictive factor). After 3 cycles he presents significant partial response (Figure 3) and continues until 6 cycles. After 10 cycles it is considered whether to stop paclitaxel and follow on with cetuximab, but given the good tolerance they remain both of them. However, in January 2018, he presented a new pulmonary progression, so we decided to start a new strategy with inmunotherapy (Nivolumab), receiving 2 cycles to date.
a. The concept of fragility is evaluated incorrectly through
the Performance Status (PS). The ACE scale 27 assesses
comorbidities and compares survival to having an advanced
stage, so that is a fact to take into account more than the ECOG
at the time of choosing the treatment.
b. When a patient progress to chemoradiotherapy, we have two good “palliative chemo” options: phase III EXTREME study(5-FU + Cisplatin + Cetuximab) or phase II of Hitt (Paclitaxel-Cetuximab) with fewer side effects, which is an alternative to cisplatin, achieving good response rates (20.43%) [1].
c. After 6 cycles of Paclitaxel + Cetuximab can be considered to keep Cetuximab as monotherapy, continue both or suspension until progression.
d. If pulmonary metastatic disease is controlled for> 1 year, we can consider surgical intervention.
e. Support treatment improves tolerance to chemotherapy. The indication of prophylactic enteral nutrition is a controversial issue, so we have to individualize.
f. We need predictive factors for each tumor type that we can know about before hand the prognosis and guide the treatment according to it. We must raise multidisciplinary strategies with the aim of achieving the best treatment sequence to improve survival in a population with few therapeutic options [2]
For more Lupine Publishers Open Access Journals Please visit our website:
http://lupinepublishers.us/
For more Open Access Journal of Oncology and Medicine Please Click Here:
https://lupinepublishers.com/Cancer-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Abstract
Clinical Case
Physical Examination: Weight 42 kg Head and Neck: mass of hard consistency of approximately 10 cm in diameter in the cervical left region that seems to deflect trachea. Pulmonary auscultation: generalized hypoventilation with some expiratory wheeze.
Additional Tests
a. Fibroscopy (August 2016) ulcerated lesion from right vallecula to mouth of Killian. Paresis of both vocal cords.b. PET-CT (August 2016): Extensive tumor in pharynxlarynx- esophagus (> 6 cm) Lymph nodes in left IB-II spaces (> 6 cm). If confirmed, carcinoma would correspond to T3 N3 (stage IVB) (Figure 1).
c. Laryngeal Biopsy: Moderately differentiated and keratinizing squamous cell carcinoma.
So, confirmed Squamous cell carcinoma stage cT4N3 Mx.The case is presented in the Tumor Committee, deciding treatment with Chemoradiotherapy and 1 cycle of Docetaxel+ Cisplatin (60% dose reduction because of frailty/ malnutrition) previous to induction due to the large tumor volume and waiting for start radiotherapy. Tracheotomy is performed prior to starting because of the risk of airway obstruction and also gastrostomy is placed for nutritional support.
On 31th August 2016 the patient starts on RT concomitantly to Cisplain ( receiving 2 cycles on 12.09.2016 and 10.10.2016 and 70 Gy) During the treatment, he achieves a good general condition until January 2017, when he goes to Otolaryngology Clinics referring dysphagia again although he maintains weight in 51 kg. PET-CT is performed: disease progression with soft tissue increase in the pharyngoesophageal junction despite the good response of cervical adenopathies and the partial response of the primary tumor. New bilateral subpleural pulmonary nodules suggestive of metastasis. Figure 2 Due to progression, he restarts treatment with chemotherapy (palliative intention) with ERBITAX scheme (Paclitaxel 80 mg / m2 weekly (3 / 4s) + Cetuximab 400 mg / m2 followed by 250 mg / m2) with good tolerance,only highlighting secondary rash to cetuximab (predictive factor). After 3 cycles he presents significant partial response (Figure 3) and continues until 6 cycles. After 10 cycles it is considered whether to stop paclitaxel and follow on with cetuximab, but given the good tolerance they remain both of them. However, in January 2018, he presented a new pulmonary progression, so we decided to start a new strategy with inmunotherapy (Nivolumab), receiving 2 cycles to date.
Discussion
b. When a patient progress to chemoradiotherapy, we have two good “palliative chemo” options: phase III EXTREME study(5-FU + Cisplatin + Cetuximab) or phase II of Hitt (Paclitaxel-Cetuximab) with fewer side effects, which is an alternative to cisplatin, achieving good response rates (20.43%) [1].
c. After 6 cycles of Paclitaxel + Cetuximab can be considered to keep Cetuximab as monotherapy, continue both or suspension until progression.
d. If pulmonary metastatic disease is controlled for> 1 year, we can consider surgical intervention.
e. Support treatment improves tolerance to chemotherapy. The indication of prophylactic enteral nutrition is a controversial issue, so we have to individualize.
f. We need predictive factors for each tumor type that we can know about before hand the prognosis and guide the treatment according to it. We must raise multidisciplinary strategies with the aim of achieving the best treatment sequence to improve survival in a population with few therapeutic options [2]
For more Lupine Publishers Open Access Journals Please visit our website:
http://lupinepublishers.us/
For more Open Access Journal of Oncology and Medicine Please Click Here:
https://lupinepublishers.com/Cancer-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Tuesday, August 27, 2019
Lupine Publishers: Lupine Publishers| Some Aspects of Red Special Win...
Lupine Publishers: Lupine Publishers| Some Aspects of Red Special Win...: Lupine Publishers- Environmental and Soil Science Abstract Due to increasing environmental radionuclide background, the scien...
Monday, August 26, 2019
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...: Lupine Publishers | Journal of Veterinary Science Abstract This paper is the first part of a three (3) part series of review...
Saturday, August 24, 2019
Lupine Publishers: Lupine Publihsers | The Development Fortified Pan ...
Lupine Publishers: Lupine Publihsers | The Development Fortified Pan ...: Lupine Publishers | Journal of Veterinary Science Abstract The main objective of the research is to develop pan bread nutri...
Lupine Publishers | Micro-Environmental Systems and Endothelial Cells in Cooperative Tumorigenesis Account for Potential Malignant Transformation in Neurofibromatosis Type 1 Patients
Lupine Publishers | Open Acess Journal Of Oncology and Medicine
Overall tumorigenesis in neurofibromatosis type 1 patients
constitutes a series of specific targeting events with a central role
enacted by proliferation of fibroblasts and endothelial cells in
overproduction of growth factors and cytokines such as transforming
growth factor-beta and CXCL12 cytokine. The plexiform neurofibroma
well-illustrates dimensions of such cooperative participation
within operative fields of the initial Schwann cell proliferation
leading in a significant number of patients to malignant transformation
of the peripheral nerve sheath tumors. Inclusive directions in operative
targeting of Schwann cells or astrocytes are staged
performance in the transformation of hyperproliferative induction and
constitute further evolutionarily defined incorporation of
such systems as endothelial cells. Hyperproliferative cell subsets are
initial and also consequential target formulation of potential
malignant states as induced in malignant peripheral nerve sheath tumors.
Neurofibromatosis type 1 (NF1) is a neurogenetic disorder and
involves both heterozygous and homozygous absence/reduction
of neurofibromin that acts normally as a tumor suppressor.
There is a need to assess predisposing genetic factors and loss
of heterozygosity causing emergence of aggressive neoplasms
in patients with NF1 [1]. The two hit hypothesis helps account
for the emergence of Schwann cell-based proliferations and
for neurofibromas and plexiform neurofibromas. Gherkin may
act on tumorigenesis of cutaneous neurofibromas via growth
hormone secretagogue receptor [2]. It is important to consider the
neurofibroma that is based on micro-environmental potentiation of
tumor generation in patients that develop malignant nerve sheath
tumors and astrocytomas in patients with NF1 +/- genotype;
this occurs in a manner that involves growth factor overactivity
and mast cell and endothelial overactivity within a milieu that
dysfunctionally stimulates tumorigenesis. Reactive oxygen
species overproduction lead to epithelial-mesenchymal transit in
patients with neurofibromin deficiency and plays a crucial role
in NF1 tumor growth [3]. RAS activation alone is not sufficient
for malignant transformation of peripheral nerve sheath tumors;
signal transduction may potentially help identify therapies for this
neoplasm type [4].
The dynamics of neurofibromin as a cytoplasmic protein involve
the regulation of K-Ras, and the PI3K/Akt pathways; absence of
neurofibromin leads to overactivation of these pathways in various
ways in inducing tumorigenesis in such lesions as optic tract
pilocytic astrocytomas, brain stem astrocytomas and also other
CNS astrocytomas in terms of progression of these lesions. The cell
of origin determines the temporal course of neurofibromatosis-1
low-grade glioma formation [5]. The micro-environment of
plexiform neurofibromas of peripheral nerves and of nerve plexi
include a 10% risk of malignant change with subsequent aggressive
clinical behavior in the affected patients. Over expression of cellular
retinoid acid binding protein 2 is reported in several cancer types,
including malignant peripheral nerve sheath tumors (MPNSTs) [6].
The neurofibromin insufficiency status in Schwann cells
and fibroblasts allows for enhanced participation of immune
system component cells such as microglia as evidenced in optic
pathway low-grade astrocytomas. Telomere erosion is described
in many tumor types and may potentially drive genomic
instability and clonal progression in NF1-associated MPNSTs
[7]. Tumor dimensions include proliferation of astrocytic cells
in optic pathways, and of various subtypes of stromal cells such
as fibroblasts and mast cells in the peripheral nervous system. It
is significant to consider particularly the micro-environmental
active participation in the genesis of the most common tumor type
in Neurofibromatosis type 1 patient, that is the neurofibroma,
which invokes proliferation of fibroblasts and endothelial cells.
The congenital plexiform neurofibroma is in fact a hypervascular
lesion that transgresses tissue margins and induces a significant
risk for malignant transformation. NF1 loss is the primary driver
of tumorigenesis in neurofibromatosis type 1-related plexiform
neurofibroma [8]. It is further to such considerations that important
cooperative intervention in malignant transformation of plexiform
neurofibromas invokes multi-type cells in inducing proliferation of
an integral Schwann cell-fibroblastic twin population in enhancing
potential malignant transformation of the peripheral nerve sheath.
A therapeutic window for neuroprotective intervention exists
as detected by optical coherence tomography in mice with optic
glioma, and particularly as an accurate biomarker of retinal ganglion
cell apoptosis [9]. The heterozygous absence of one neurofibromin
allele in mice results in plexiform neurofibromas and low-grade
optic pathway astrocytomas. Mast cells appear to play a causal role
in neurofibroma formation and also in microglia in optic pathway
glioma evolution [10]. Such implications of the micro-enviromental
factors includes a distinctive cooperative participation that carries
implications for significant enhancement of cell proliferation
and of such cytokines such as transforming growth factor and
CXCL12 in formulating malignant transformation in such tumors.
The methylemetetrahydrofolate reductase 1298 and 677 gene
polymorphisms are related to optic glioma and hamartoma risk
in NF1 patients through effects on DNA synthesis and methylation
[11].
The related tuberous sclerosis complex is analogous to
neurofibromatosis type 1 as a neurogenetic disorder associated
with increased risk for astrocytomas in the form of subependymal
giant cell astrocytomas. A convergent targeting of systems of cell
proliferation include in particular cyclic AMP and Ras in a manner
that includes dimensions of micro-environmental conditioning.
Mutations of the NF 1 gene are frequent in many cancer types
in patients without NF1 and this is suggestive of a more general
role for the NF1 gene in oncogenesis. In melanoma NF1 mutations
potentially drive tumorigensis and promote drug resistance [12].
Inclusive dynamics allow for permissive tumorigenesis in a manner
that includes the incorporation of malignant transformation within
confines of a Schwann cell-fibroblast-endothelial cell system in
the case of malignant peripheral nerve sheath tumors. Astrocytes
and microglia are analogous counterparts in the induction of
CNS astrocytomas. Such considerations are inclusive phenomena
of multi-component induction of potential malignancy that recharacterizes
conditioning of the micro-environment of proliferative
states preceding tumorigenesis. Interaction between neoplastic
Schwann cells and their surrounding neural microenvironment
has important implications for early cellular events promoting
tumorigenesis in neurofibroma development [13].
Performance dynamics of tumors in neurofibromatosis type
1 may potentially modify the biologic significance of a two-hit
hypothesis in a manner that implicates micro-environmental
conditioning of the resultant cell hyperplasias and proliferations in
such lesions as peripheral nerve sheath tumors and astrocytomas.
NF1 provides unique vantage points to examine co-contributions
of molecular, cellular, and tissue processes in tumor biology [14].
Such proposed dimensions invoke in particular an over-activation
in production and action of growth factors that provoke selective
malignant transformation of hyper-proliferative lesions composed
of Schwann cells and astrocytes in the peripheral and central
nervous systems respectively. Plasma soluble levels of transforming
growth factor-beta and interleukin-6 are increased in NF1 patients
and a shift towards an anti0inflammatory profile has been reported
in cells expressing cytokines [15].
The hyperproliferative states affecting Schwann cells and
astrocytes invoke also fibroblast and microglial cell proliferations
in a manner transforming tumorigenesis. Such facilitation to
tumorigenesis invokes dimensions of transformation as well
seen in plexiform neurofibromas that may undergo malignant
transformation in a significant number of affected individuals.
Such considerations are selective targeting of specific cell subpopulations
in a manner that allows permissive transformation.
Insertional mutagenesis identifies a STAT3/Arid1b/beta-catenin
pathway that drives neurofibroma initiation in the context of Nf1
loss [16]. Mast cells and fibroblasts may potentially incorporate
endothelial cells that may participate as central dysregulatory
dimensions in plexiform neurofibroma tumorigenesis. The
provocations for malignant transformation further cooperate
in systems of derivative consequence as hypervascular lesions
that subsequently lead to potential malignant cells in individual
patients. Cross species comparative oncogenomic may identify
driver mutations in mouse cancer models and allow validation in
human tumors [17].
Propositional implications in tumorigenesis include the
multi-component participation of Schwann cells on the one hand and of fibroblasts, mast cells, endothelial cells and also of
microglia in an inductive process that includes specific pathways
of malignant transformation. Endothelial cell proliferation is
related to substantial participation in modes related to key-events
of increased proliferation of Schwann cells and astrocytes in initial
stages of lesion infliction. Inclusive phenomena have thus become
systems of consequence in affecting such specific cell proliferative
states. Such events occur within the added dimensions of directed
targeting of multiple-agent micro environmental modeling of the
initial proliferation of the Schwann cells or astrocytes. A pivotal
series of roles played by fibroblasts, endothelial cells, mast cells
and of microglia and astrocytes appears a dynamic milieu within
added consequences of malignant transformation of both Schwann
cells and astrocytes that progress as cooperative systems of
tumorigenesis.
Abstract
Introduction
Neurofibromin
Related Tumor Predispositions
Convergent Targeting
Performance Dynamics
Hyperproliferation
Concluding Remarks
For more Lupine Publishers Open Access Journals Please visit our website:
http://lupinepublishers.us/
For more Open Access Journal of Oncology and Medicine Please Click Here:
https://lupinepublishers.com/Cancer-journal/
http://lupinepublishers.us/
For more Open Access Journal of Oncology and Medicine Please Click Here:
https://lupinepublishers.com/Cancer-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
0
Friday, August 23, 2019
Lupine Publishers: Lupine Publishers | Use of Dietary Yeast and its P...
Lupine Publishers: Lupine Publishers | Use of Dietary Yeast and its P...: Lupine Publishers | Journal of Veterinary Science Abstract All around the world, sheeps and goats play an important role in ...
Thursday, August 22, 2019
Lupine Publishers: Major Issues Related to Women Health, Social, Cult...
Lupine Publishers: Major Issues Related to Women Health, Social, Cult...: Journal of Gynaecology | Lupine Publishers Abstract Present article sketches out major issues related to health, social...
Wednesday, August 21, 2019
Lupine Publishers: Optimization of Chitosan+Activated Carbon Nanocomp...
Lupine Publishers: Optimization of Chitosan+Activated Carbon Nanocomp...: Journal of Chemical Sciences | Lupine Publishers Abstract First, the minimum energy (geometry optimization DFT-DMol3) i...
Tuesday, August 20, 2019
Lupine Publishers: Bowels and Urine Odors and Its Solutions | Lupine ...
Lupine Publishers: Bowels and Urine Odors and Its Solutions | Lupine ...: Journal of Diabetes and Obesity | Lupine Publishers Opinion From the beginning time people go for bowel outside in the fi...
Monday, August 19, 2019
Lupine Publishers: Lupine Publishers | Sicilian Lemon and Honey Light...
Lupine Publishers: Lupine Publishers | Sicilian Lemon and Honey Light...: Lupine Publishers | Journal of Veterinary Science The development of products enriched with physiological components such as pro...
Wednesday, August 14, 2019
Lupine Publishers: Eating Disorders in Developing Countries | Lupine ...
Lupine Publishers: Eating Disorders in Developing Countries | Lupine ...: Open Access Journal of Neurology | Lupine Publishers Editorial Eating disorders, once thought to be a set of rare disea...
Tuesday, August 13, 2019
Lupine Publishers: The Physical Study of Vertical Structure of Temper...
Lupine Publishers: The Physical Study of Vertical Structure of Temper...: Journal of Oceanography | Lupine Publishers Abstract Always oceanographers pay attention to layer structure in the sea environme...
Thursday, August 8, 2019
Lupine Publishers: Lupine Publishers | Microbial Source Tracking Mark...
Lupine Publishers: Lupine Publishers | Microbial Source Tracking Mark...: Lupine Publishers | Journal of Veterinary Science Microbial source tracking (MST) describes a suite of methods and an investigat...
Wednesday, August 7, 2019
Lupine Publishers: Metals Phytotoxicity Assessment and Phyto Maximum ...
Lupine Publishers: Metals Phytotoxicity Assessment and Phyto Maximum ...: Journal of Chemical Sciences | Lupine Publishers Abstract In this paper, the influence of metals (Cd, Pb, Cu, Co, Ni, Z...
Tuesday, August 6, 2019
Lupine Publishers: Experimenting with New Crops at the Peri-Urban Fri...
Lupine Publishers: Experimenting with New Crops at the Peri-Urban Fri...: Agriculture open access journals | Lupine Publishers Farming at the Peri-Urban Fringe As the world’s human population ...
Friday, August 2, 2019
Lupine Publishers: Lupine Publishers | Molecular Typing Of Capsular P...
Lupine Publishers: Lupine Publishers | Molecular Typing Of Capsular P...: Lupine Publishers | Journal of Veterinary Science Abstract Forty five Staphylococcus aureus isolated from cases of bovin...
Subscribe to:
Posts (Atom)
Lupine Publishers | Open Access Journal of Oncology and Medicine (OAJOM)
Thanksgiving is a national holiday celebrated on various dates in the United States, Canada, Grenada, Saint Lucia, and Liberia. ...

-
Lupine Publishers: Eating Disorders in Developing Countries | Lupine ... : Open Access Journal of Neurology | Lupine Publishers Edi...
-
Lupine Publishers: Lupine Publishers | Persistent Wound Leakage After... : Lupine Publishers | Journal of Orthopaedics Abstract ...
-
Thanksgiving is a national holiday celebrated on various dates in the United States, Canada, Grenada, Saint Lucia, and Liberia. ...