Lupine Publishers Cancer Journal
Thursday, November 25, 2021
Lupine Publishers | Open Access Journal of Oncology and Medicine (OAJOM)
Monday, August 9, 2021
Lupine Publishers | Advances in Treating Relapsed Diffuse Large B Cell Lymphoma Treatment
Lupine Publishers | Open Access Journal of Oncology and Medicine
Abstract
Diffuse large B cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL), comprising about 25% of all mature NHL. First-line therapy cures about 40-60% of patients. High dose chemotherapy followed by autologous stem cell transplant can cure about 50% of patients at relapse. Transplant-ineligible patients have shorter survival with every line of subsequent therapies with a median overall survival (OS) of 10 months at second line and 4.7 months at fourth lines of therapy. There is unmet need to treat patients with relapsed DLBCL. Chimeric antigen receptor T-Cells (CAR-T), with now three FDA- approved products to treat relapsed DLBCL, provide a cure in about 40% of patients. Other recently approved agents include antibody-drug conjugate targeting CD79b (Polatuzumab Vedotin), Anti-CD19 antibodies (Tafasitamab-cxix), and nuclear export inhibitor XPO1 (Selinexor) have provided a hope to patients with relapsed DLBCL. We will discuss these new approvals with comparison of response rate and side effect profiles.
Keywords: Diffuse Large B Cell Lymphoma; CAR-T Cell Therapy; Tafasitamab; Relapsed Refractory Lymphoma; Polatuzumab
Introduction
Non-Hodgkin lymphoma (NHL) is the eighth cause of cancer death. With an estimated 81,560 new cases and 20,720 deaths in 2021 [1] DLBCL is the most common type of NHL comprising about 25% of all NHL cases [2].
The prognosis depends on different factors at diagnosis including histological subtypes (Germinal center (GC) type versus non-GC subtypes) [3], genetic subtypes (double HIT) [4], patient age, stage of the disease, extra nodal involvement, and elevated LDH (IPI scoring) 5. Other important prognostic factors can be assessed after starting therapy, includes response at interim PET scan (PET scan after 2-4 cycles of therapy)6. Initial therapy for DLBCL remained the same for all different prognostic factors and different risk group which is the famous combination of chemotherapy R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone) despite multiple attempts to try to find a better alternative [7-9]. Except for high grade DLBCL with c-Myc and BCL2 and/or BCL6 gene rearrangement which most centers comfortable with using R-EPOCH chemotherapy regimen [10]. With the stander of care R-CHOP, about 30% of diffuse large B cell lymphoma patients relapse within the first 5 years [11]. Poor response to initial therapy and, or very early relapse (within the first six months), or what is called refractory disease is one of the worse predictors of poor survival based on data from scholar-1 study [12].
Current stander of care at relapse including high dose chemotherapy followed by autologous stem cell transplant [13]. In patient with very high-risk relapse (relapse within 1 year of therapy or refractory patients) there is a suggestion that early therapy with CAR-T cell may improve outcome. this question will be answered once we receive data from the now completed ZUMA7, and BELINDA and TRANSFORM trials comparing different CAR-T cell products (axicabtagene, Tisagenlecleucel, Lisocabtagene respectively) to the current stander of care i.e., high dose chemotherapy followed by autologous stem cell transplant in this very high-risk population. Treatment after relapse from second line of therapy inpatient who continue to maintain reasonable performance status is CAR-T cell therapy. Different CAR-T cell products are currently FDA approved to treat relapsed DLBCL after failure of at least two lines of therapy including Axicabtagene ciloleucel (Axi-Cel), Tisagenlecleucel and Lisocabtagene maraleucel(liso-cel). CAR-T provide about 40-55% complete response rate (CR). Most worrisome Toxicity including cytokine release syndrome (CRS) and neurological toxicity [14-16]. See Table 1 for comparing of different CAR-T cell products. Of note patient with DHL and DEL benefited from CAR-T cell therapy at relapse with Best overall response (ORR) was 56% (10/18) for DEL patients, 50% (5/10) for DHL patients which was not different from non-DHL/DEL in a retrospective data evaluation4. In patient who are intolerable to transplant or are ineligible for CAR-T therapy or had a relapse after CAR-T cell therapy there is no stander approach. Recent advances in newer agent gave some hope in managing these patients with improving survival.
Polatuzumab Vedotin
Polatuzumab Vedotin (Pola) is an antibody-drug conjugated that bind to CD79b which is present in all B-lymphocytes including mature malignant B-Cells delivering the microtubule inhibitor (MMAE) causing direct cytotoxicity. Polatuzumab alone or in combination with anti-CD20 antibody had a modest response with CR ranging from 0-13% [17-18]. The combination of Polatuzumab, Bendamustine and Rituximab was approved by FDA for patients with relapsed DLBCL after failure of at least 2 lines of therapy. In phase II randomized study comparing Pola BR to BR alone. The median age of patient on Pola BR arm was 67, 25% had prior stem cell transplant and 75% were refractory to prior line of therapy. ORR was 63% (25 of 40 patients) with CR rate of 40%. 48% of patients remained in CR at 1 year. Common side effects included fatigue, infusion reaction, neuropathy, and pancytopenia and increase risk of infection [19].
Tafasitamab
Tafasitamab (Tafa) is a Fc-enhanced, humanized, anti-CD19 monoclonal antibody. its FC portion is enhanced by modulating two amino acids that leads to increase affinity to Fcy receptors. As single agent Tafa has a response in DLBCL of 26% with responses lasting >12 months [20]. Phase II L-MIND study studied the combination of Tafa with lenalidomide as previous in vitro studies showed synergetic activity [21]. The study showed impressive CR rate of 43% with median duration of response of about 34 months. To compare the data from Pola-BR and Tafa-Lenalidomide combinations refer to (Table 2).
Selinexor
Selinexor is an XPO1(exportin 1) inhibitor. XPO1 is responsible for removal of multiple tumor suppressor genes out of the nucleus like P53, P73 and IkBk outside nucleus and inhabiting their function. The FDA had approved Selinexor as a single agent for the treatment of relapsed or refractory DLBCL after at least 2 lines of therapy. The approval was based on SADAL study, which was a Phase II study that included 134 patients, 45% were 70 years or older, 4% with DHL,41% had more than 3 lines of previous therapy with 72% refractory to the last line, 13% had elevated LDH. Single agent Selinexor resulted in ORR of 29%(95%CI:22,38) with CR rate of 13% with response duration of 6 months. Most common adverse reaction was fatigue, gastrointestinal side effects and pancytopenia [22]. Preclinical data suggest that the inhibition of XPO1 can provide a therapeutic target for DHL [23-24].
Bispecific T cell engager
Bispecific T-Cell engager (BiTe) therapy provide a promising off the shelve immune therapy for multiple cancers including NHL. In the contrary to CAR-T cell therapy, BiTe does not require manufacturing time or pre-infusion conditioning chemotherapy. Side effect can be similar but potentially less severe than CAR-T including CRS and neurological toxicity [25]. At this time BiTE therapy is not FDA approved to treat NHL but multiple trials across the world are testing different BiTe as single agent or with different combination including combining BiTe with Polatuzumab, with lenalidomide or other combinations. Recently published phase I/ II trial with Glofitamab, Bivalent CD20-target T-cell engaging BiTe. The study included 171 patients, 90% refectory to prior lines of therapy with median of 3 previous lines of therapy, 74% had DLBCL. ORR was in the phase II dose was 65.7% with CR of 57.1%. 84% of Cr patients had a maximum of 27.4 duration of response. Grade ¾ CRS was seen in 3.4% and grade 3 CNS toxicity in 1.2% [26]. Other promising BiTes including Blinatumomab, Epicoritamab, Monsunetuzumab and others [27-30].
Immune-Checkpoint Inhibitors in lymphoma
Immune check point inhibitors including programmed cell death /ligand inhibitors PD1 and PD-L1 inhibitors and CTL4 inhibitors have so far limited role in the therapy of DLBCL in the absence of biomarker targeted therapy. Future use may include pretesting for PD1 expression or the use of checkpoint inhibitors with combination [31].
CD47 Inhibitors
CD47 is present in virtually all cancer cells and it over expression is associated with poor prognosis. It is an inhibitory signal to macrophages (Do not Eat me signal) and inhabitation of CD47 lead to potentially increasing macrophage activation and tumor destruction. In addition, macrophages will present more tumor antigen and increase T-cell mediated cytotoxicity [32]. In Phase I/II trial combining CD47 inhibitor 5F9 with rituximab, the study included 22 patients (15 had DLBCL). Among patients with DLBCL, objective response rate was 40% with CR rate of 33%. Most common side effects were anemia and infusion reaction [33].
Conclusion and opinion
The treatment of relapsed DLBCL remains a challenge. With the approval of now three CAR-T cell therapy product we do have a chance of curing at least a third of those patients. Other may not benefit or may not have access to CAR-T cell therapy. Recently approved regimens that provide a good promise are the Tafa and lenalidomide combination and polatuzumab with Rituximab and Bendamustine combination. Although we have some patients with prolonged responses these combination does not provide a cure as of now. And most patients eventually succumb to their disease. With sequencing different combination and possibly prolonged maintenance therapy as, possible with the use of BiTE or other targeted agents we may see in the future an improved survival of relapsed refractory lymphoma.
Read More Lupine Publishers Oncology and medicine Articles :
Read More Lupine Publishers blogger Articles :
Tuesday, May 26, 2020
Lupine Publishers | A Case Report and Review of Thymic Carcinoma with Adenoid Cystic Carcinoma like Features
Introduction
Information and history
Discussion

The Character of Clinical
The Character of Pathology
The Differential Diagnosis
The treatment and prognosis
The discussion of pathogenesis
Read More Lupine Publishers Oncology and medicine Articles :
Read More Lupine Publishers blogger Articles :
Wednesday, May 13, 2020
Lupine Publishers | Promising Role of Fractional Calculus in Biomedicine and Biophysics
where Γ denotes the Gamma function and , . And its Laplace transform can be given by:
Where C is the set of complex numbers? It is worth mentioning that the exponential function is just a special case of α = l Mittag-Leffler function, for example for the special case of , the Mittag-Leffler function Eq. (3) reduces to the exponential function E1(z) = ez . This point is very important because of that the natural exponential function has been considered as a fundamental function of natural science and in particular biology up to now, so that many phenomena could be described using it and now scientist are able to think that with such this new framework (i.e. fractional differential equations and their solutions in terms of Mittag-Leffler functions) they can find many new results and information about biological and biomedical phenomena [2,3].
Thursday, March 12, 2020
Lupine Publishers: Lupine Publishers | Principles of the Military Con...
Lupine Publishers | Acute Erythroblastic Leukemia Revealed by Dermatological Manifestations
Abstract
Case Report
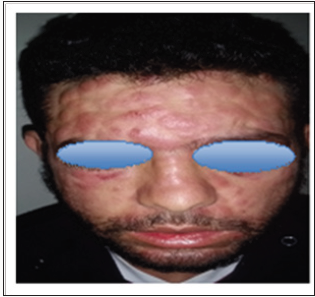
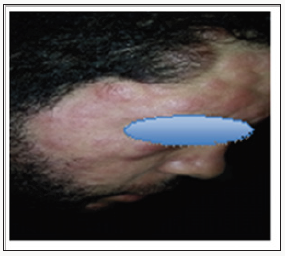
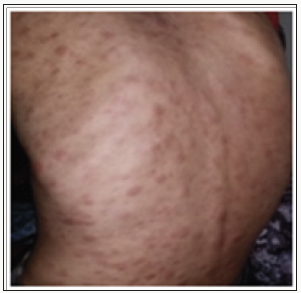
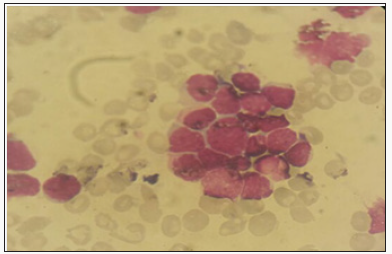
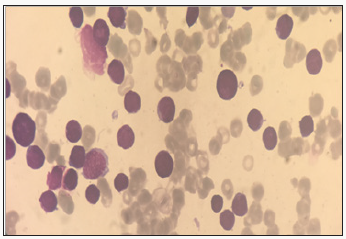
Discussion
The specific dermatological lesions which can reveal hematological diseases [4], are mainly represented by leukaemides (leukaemia cutis), which are red brown to purple dermal papules, plaques or nodules. Granulocyticsarcom as an extra-medullary tumour masses, ulcerated plaques and gingival hypertrophy [5]. The infectious dermatoses secondary to the biological disturbances accompanying the malignan themopathy and their treatments. The occurrence of specific cutaneouslesions in leukemia is synonymous with a major aggravation of the prognosis (with for example a survival twice as short if there is a specific cutaneous involvement); this seriousness make some authors propose different treatments with a medium-long stay hospitalization[6-8].
In our case, acute myelonlastic leukemia 6 (AML 6 ) was revealed by diffuse leukemias resulting from the infiltration and proliferation of malignantha ematological cells (blasts) in the skin and by gingival hyperplasia secondary to mucosal infiltration [5]. The clinical presentation of acute leukemia including AML6 in the form of ulcer ativenecroticgingivitis in the foreground, is a rare form to be remembered, mentioned in all courses of medicine and dentistry, stipulating that Gingival involvement is a classic feature of leukemia [6] The frequent association of skin cancers with haematological malignancies is also highlighted in several publications [5].
Conclusion
For More Lupine Publishers Open Access Journals Please visit our Website:
For more Open Access Journal of Oncology and Medicine Please Click Here: https://lupinepublishers.com/cancer-journal/
Thursday, February 27, 2020
Lupine Publishers | Subjection between Breast Cancer and Body Mass Index, the Role of L-Carnitine in Prediction and Outcomes of the Disease
Abstract
Keywords: Body mass index (BMI); Breast cancer (BC); Obesity; Overall survival; L carnitine
Introduction
Obesity has a chronic metabolic character, which is the result of the interaction of the endogenous factors, environmental conditions and lifestyle. Endogenous factors could be considered a violation of the genetic and hormonal balance. The external conditions and type of lifestyle include irregular rhythm nutrition, use of substandard products and sedentary lifestyle. Obesity is the first risk factor for metabolic syndrome, diabetes type II, cardiovascular disease and some forms of cancer, including breast cancer. Since overweight is a risk factor for breast cancer, there is reason to believe that among patients with breast cancer the percentage of obese women is higher than in the population. The risk of breast cancer in postmenopausal women by 30%, it is more than in premenopausal, women with obesity-50%. Furthermore it was proven that obesity is associated with poor prognosis in patients with breast cancer, regardless of menopausal status, and effectiveness of systemic medication breast cancer in patients that have over weight is lower than in patients with normal BMI.
Although obesity is associated with a poor outcome in women with breast cancer, it is unclear how weight loss after diagnosis will change its course and results. Recently, complementary and alternative medicine (CAM) is widely accepted among patients with breast cancer, which may provide several beneficial effects including reduction of therapy-associated toxicity, improvement of cancer-related symptoms, fostering of the immune system, and even direct anticancer effects [2]. L-carnitine is a metabolite of C4 oil LC, which is involved in the transfer of palm-n-LC through the inner membrane into the mitochondrial matrix and is a substrate for the formation of ATP molecules. Carnitine is a trim ethylated amino acid naturally synthesized in the liver, brain and kidneys from protein lysine and methionine. Several factors, such as sex hormones and glucagon, can influence the distribution and level of carnitine in tissues [3,4].
In the absence of L-carnitine, the inner membrane of the mitochondria becomes impermeable to fatty acids, which entails a chain of various metabolic disorders in the human body. Carnitine has a modulating effect on the function of acetylcholine excitatory neurotransmitter, glutamate excitatory amino acid, insulin growth factor-1 (IGF-1) and nitric oxide (NO)[3]. Also proved, that L-carnitine may have a dual protective effect by enhancing the energy dynamics of the cell and inhibiting cell membrane hyper excitability, which make it an ideal nutrient for cancer prevention and treatment [5]. In view of the foregoing, the study of the influence of the body mass index on the effectiveness of systemic treatment of breast cancer is an urgent scientific problem and a promising field of research. This article presents the information of epidemiological and clinical studies of the influence of the body mass index on the effectiveness of breast cancer treatment by individualizing therapeutic measures taking into account the characteristics of patient's metabolism.
Studies on the Effects of BMI on The Course and Outcome of Breast Cancer and the Role of L-Carnitine in the Treatment of Cancer: The effectiveness of the prescribing of L-carnitine for breast cancers' treatment, as well as the effect of BMI on the outcome of the disease is proven in epidemiological and clinical studies.
Epidemiological and Clinical Studies
DSM Chan and co-authors [6] reported that women who have BMI> 30 course and outcomes of breast cancer are significantly worse than women with BMI <30. They proved, that women with BMI> 30 have the overall relative risk of total mortality 1.41, women with BMI of 25> 30 - 1.07. At the same time, for every 5 kg / m2 of the increase BMI, the risk of both total mortality and mortality from breast cancer increased, namely by 18% and 14%, respectively M. Protani and co-authors [7] have shown that women with breast cancer, who are suffering in obesity, have lower survival rate than women with breast cancer without obesity. Recently published data of randomized clinical researches by ML Neuhouser and coauthors [8] demonstrated, that for women> 50 years old, with 2 and 3 stages of obesity (BMI> 35) is typically the development of GR+ breast cancer.Similarly, B. Pajares et al. [9] who found significantly worse results for patients with BMI >35 compared with patients with BMI <25, stated that the magnitude of the effect depended on the cancer subtype (estrogen receptor (ER) / progesterone (PR) positive and HER2 negative, HER2 positive, triple negative). An analysis of the pooled data of the three adjuvant studies of the Eastern Cooperative Cancer Group showed significantly worse results for patients with obesity (BMI > 30) than for patients with normal BMI with a hormonal receptor-positive disease. And it was noted absence of negative effect of obesity on survival in patients with other breast cancer subtypes. C Fontanella et al. [10] studied the effect of BMI on different molecular subtypes of breast cancer and concluded that in women with ER / PR-positive and HER2-negative breast cancer, as well as with TNBC, the risk of death is significantly higher than in other subtypes of cancer.
It is proved that even the highest BMI figures are not a risk factor for death for patients with luminal A-like subtype of breast cancer. The reason for this is that fatty tissue produces an excessive amount of estrogen, a high level of which is associated with an increased risk of developing breast, endometrial, ovarian and some other cancers. It has also been proven that the level of adipokine, that promotes cell proliferation, increases in the blood with increasing of level of fat in organism. And adiponectin, which people with obesity have less than people with normal BMI, can have anti proliferative effects. Such data can serve as evidence of the effect of BMI on the course and outcome of breast cancer. Yet another proof of influence developing metabolic syndrome on the course and outcome of breast cancer was proposed by R. Bhandari et al. [11]. They proved that that the presence of metabolic disorders (that is, the metabolic syndrome) is associated with an increased risk of breast cancer in adult women.
The above data led to the need to investigate medicines that contribute to fat burning, such as L-carnitine. Based on the data provided by Rania M. Khalil and co-authors [12], we can prove the positive effect of this medicine on the course and outcome of breast cancer. The study showed that patients who received Tamoxifen with L-carnitine had significant decrease of Her-2 / neu and IGF-1 level (P <0.05) in the serum compared with patients who received only Tamoxifen. Using of L-carnitine led to significant decrease Her- 2 / neu level in the serum (P <0.05) compared to each of the control patients, namely, 59.5%. The effect of tamoxifen on IGF-1 (P <0.05) -decrease its level by 5.4% [13].However, it has been proved that using of L-carnitine in the treatment of ER+ breast cancer does not significantly reduce the level of estradiol, but leads to decrease both tumor markers CEA and CA15.3 (P <0.05,% decrease by 80.9% and 67, 8%, respectively) [13].
Using of L-carnitine in patients with breast cancer and obesity improves the metabolism of fatty acids in mitochondria, restores normal mitochondrial function and, thus, improves the general condition and quality of patients’ life [14]. Carnitine may alsomimic some of the biological activities of glucocorticoids, particularly immunomodulation, via suppressing TNF-a and IL-12 release from monocytes (5). L-carnitine as adjuvant therapy in cisplatin-treated cancer patients proved a beneficial effect in reducing the cisplatin- induced organ toxicity [15]. It is possible that, the extremely lipophilic nature of carnitine may be responsible for the decrease in EGFbinding [16]. Carnitine may insert in the cell membrane and/or interact with one of the many cellular enzymes having lipid substrates or cofactors. In addition, carnitine may interact directly with the EGFR [17].
Experimental evidence is available showing that ROS may induce the light and independent phosphorylation of the EGFR activating Her-2/neu. Moreover, the expression of the receptor is induced in conditions of oxidative stress [18]. L-carnitine, via its free radical scavenging and antioxidant properties, may inhibit ROS-mediated EGFR phosphorylation. It has been found that palmitoyl-carnitine can inhibit the activity of heart and brain protein kinase C in a competitive manner and subsequent phosphorylation of the EGFR [19]. Although the tumor markers and IGF-1 showed no significant difference in TAM-treated patients before and after administration of L-CAR, there was a tendency to decline after L-CAR supplementation [13]. The results of the above studies became a prerequisite for conducting clinical studies aimed at establishing the role of L-carnitine in the treatment of breast cancer.
To date, the search in the online clinical research registration system ClinicalTrials.gov using key words L-carnitine + breast cancer has revealed several studies evaluating the efficacy and safety of L-carnitine in the treatment of breast cancer patients. Analyzing the obtained results, we can conclude that L-carnitine was the drug of choice for neuropathies, as a consequence of chemotherapy, in patients with breast cancer.
Conclusion
https://lupinepublishers.com/cancer-journal/fulltext/subjection-between-breast-cancer-and-body-mass-index-the-role-of-l-carnitine-in-prediction-and-outcomes-of-the-disease.ID.000103.php
Lupine Publishers | Open Access Journal of Oncology and Medicine (OAJOM)
Thanksgiving is a national holiday celebrated on various dates in the United States, Canada, Grenada, Saint Lucia, and Liberia. ...

-
Lupine Publishers | Open Access Journal of Oncology and Medicine Introduction The study of complex systems and investigation of th...
-
Lupine Publishers | Open Access Journal of Oncology and Medicine Abstract Diffuse large B cell lymphoma (DLBCL) is the most c...
-
Thanksgiving is a national holiday celebrated on various dates in the United States, Canada, Grenada, Saint Lucia, and Liberia. ...







